Ir Spec Table

The infrared (IR) spectroscopy is a powerful analytical technique used to identify and characterize the molecular structure of a sample. One of the key components of IR spectroscopy is the IR spectrum, which is a plot of the absorption of infrared radiation by a sample as a function of wavelength or frequency. The IR spectrum is typically displayed as a table or chart, with the x-axis representing the wavelength or frequency and the y-axis representing the absorbance or transmittance.
Infrared Spectroscopy Table
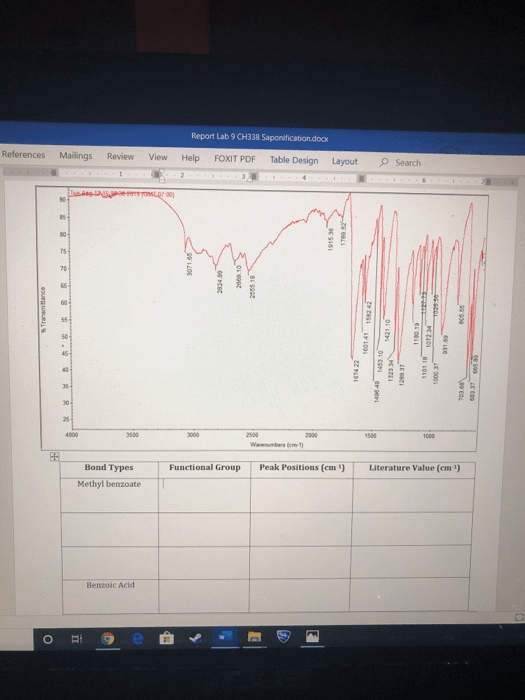
The IR spectroscopy table, also known as the IR spec table, is a comprehensive table that lists the characteristic IR absorption bands for various functional groups. The table is used to identify the functional groups present in a sample and to determine the molecular structure of the sample. The IR spec table is typically organized by functional group, with the characteristic absorption bands listed for each group.
IR Spec Table Categories
The IR spec table is typically categorized into several sections, including:
- Alkanes and cycloalkanes
- Alkenes and alkynes
- Aromatics
- Alcohols and phenols
- Ethers and epoxides
- Aldehydes and ketones
- Carboxylic acids and derivatives
- Amines and amides
Each section lists the characteristic IR absorption bands for the corresponding functional group, along with the wavelength or frequency range and the intensity of the absorption band.
| Functional Group | Characteristic IR Absorption Bands (cm-1) |
|---|---|
| Alkanes | 2850-3000 (C-H stretch), 1350-1470 (C-H bend) |
| Alkenes | 1630-1670 (C=C stretch), 3080-3140 (C-H stretch) |
| Aromatics | 1450-1600 (C=C stretch), 3000-3100 (C-H stretch) |
| Alcohols | 3200-3600 (O-H stretch), 1000-1300 (C-O stretch) |
| Ethers | 1000-1300 (C-O stretch), 2800-3000 (C-H stretch) |
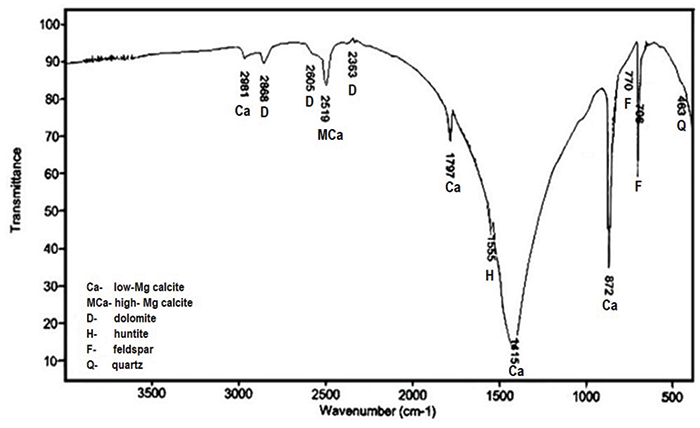
Interpretation of IR Spectra
The interpretation of IR spectra requires a thorough understanding of the IR spec table and the characteristic absorption bands for various functional groups. The following steps can be used to interpret an IR spectrum:
- Identify the characteristic absorption bands for each functional group present in the sample.
- Determine the wavelength or frequency range for each absorption band.
- Compare the observed absorption bands with the characteristic absorption bands listed in the IR spec table.
- Assign the functional groups present in the sample based on the characteristic absorption bands.
By following these steps and using the IR spec table, it is possible to determine the molecular structure of a sample and identify the functional groups present.
What is the IR spec table used for?
+The IR spec table is used to identify the functional groups present in a sample and to determine the molecular structure of the sample.
How is the IR spec table organized?
+The IR spec table is typically organized by functional group, with the characteristic absorption bands listed for each group.
What information is included in the IR spec table?
+The IR spec table includes the characteristic IR absorption bands for various functional groups, along with the wavelength or frequency range and the intensity of the absorption band.
