Ir Spectrum Functional Groups
The infrared (IR) spectrum is a powerful tool used in organic chemistry to identify functional groups within molecules. Functional groups are specific groups of atoms within a molecule that determine its chemical properties and reactivity. By analyzing the IR spectrum of a compound, chemists can identify the presence of specific functional groups, which is essential for understanding the compound's structure and properties.
Introduction to IR Spectroscopy
IR spectroscopy is a technique that measures the absorption of infrared radiation by a molecule. When a molecule is exposed to IR radiation, it absorbs energy at specific frequencies, resulting in the vibration of its bonds. The IR spectrum is a plot of the absorption of IR radiation versus the frequency of the radiation. The frequency of absorption is related to the energy difference between the vibrational states of the molecule.
Interpreting IR Spectra
Interpreting IR spectra requires a basic understanding of the relationship between the frequency of absorption and the type of bond or functional group present. The IR spectrum is typically divided into several regions, each corresponding to a specific type of bond or functional group. The main regions of the IR spectrum are:
- 4000-2500 cm^-1: This region corresponds to the O-H, N-H, and C-H stretching vibrations.
- 2000-1500 cm^-1: This region corresponds to the C=C, C=O, and C=N stretching vibrations.
- 1500-1000 cm^-1: This region corresponds to the C-H, C-O, and C-N bending vibrations.
- 1000-500 cm^-1: This region corresponds to the C-H, C-O, and C-N stretching and bending vibrations.
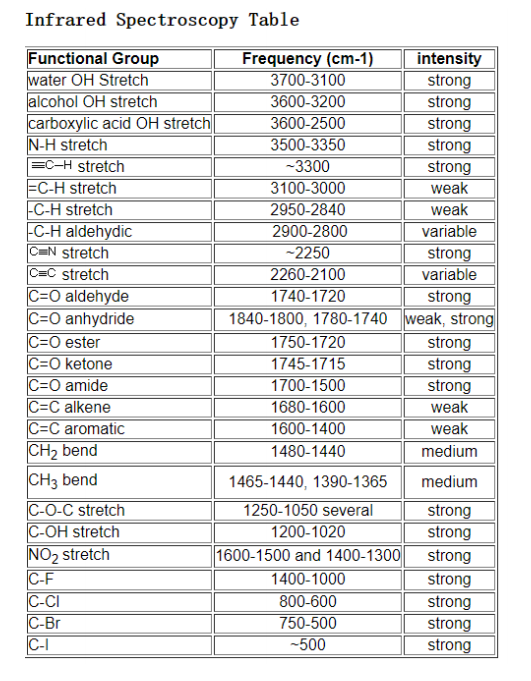
Common Functional Groups and Their IR Absorptions

The following are some common functional groups and their characteristic IR absorptions:
| Functional Group | IR Absorption (cm^-1) |
|---|---|
| O-H (alcohols) | 3600-3200 |
| N-H (amines) | 3500-3300 |
| C=O (carbonyl) | 1800-1650 |
| C=C (alkenes) | 1700-1600 |
| C=N (nitriles) | 2250-2200 |
| C-O (ethers) | 1300-1000 |

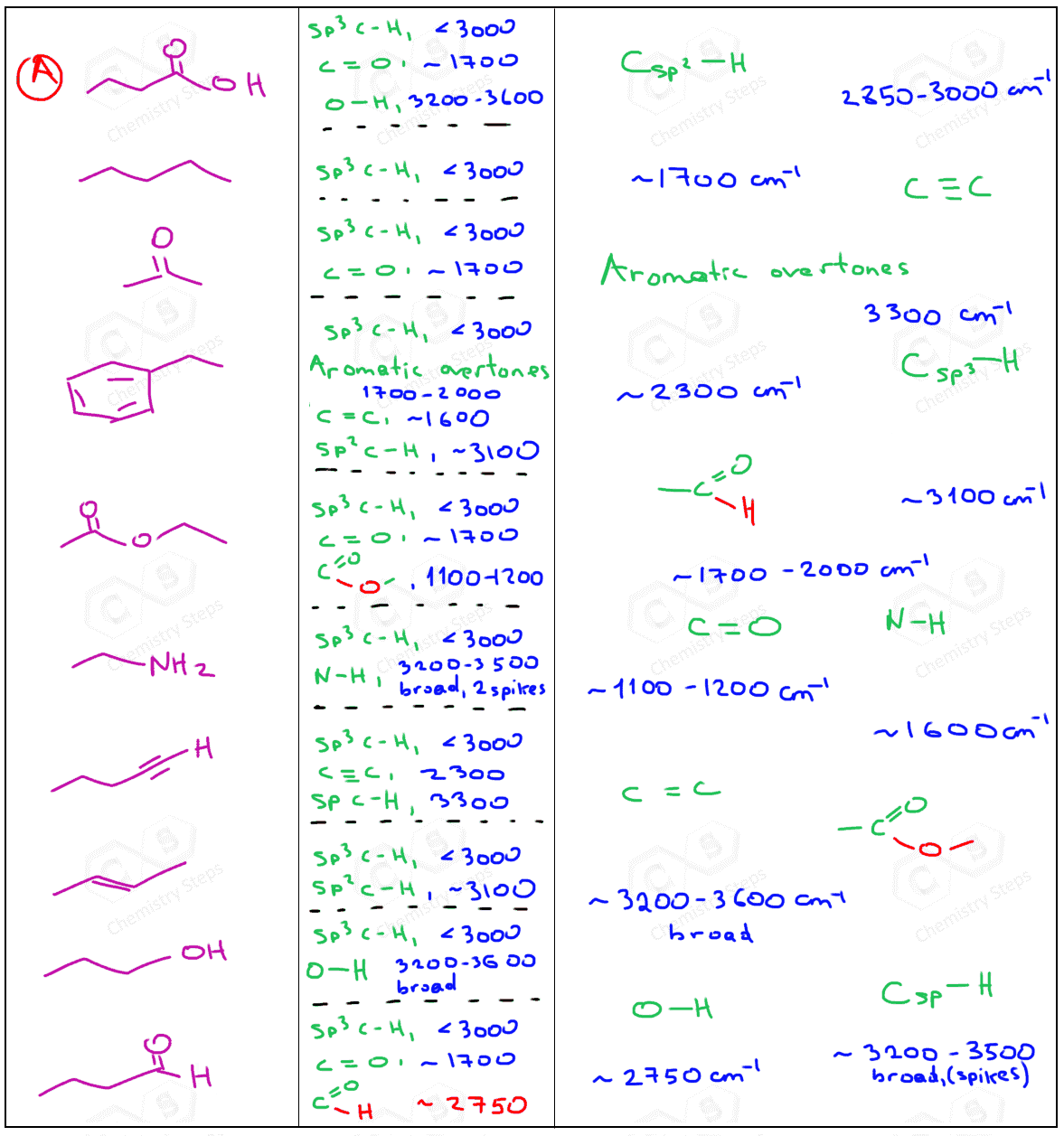
Alkane and Alkene Functional Groups
Alkanes and alkenes are two common types of hydrocarbons. Alkanes are saturated hydrocarbons, while alkenes are unsaturated hydrocarbons containing a carbon-carbon double bond. The IR spectrum of an alkane typically shows a strong absorption at around 3000-2800 cm^-1, corresponding to the C-H stretching vibration. Alkenes, on the other hand, show a strong absorption at around 1700-1600 cm^-1, corresponding to the C=C stretching vibration.
Carbonyl Functional Groups
Carbonyl functional groups, such as aldehydes, ketones, and esters, are characterized by a strong absorption at around 1800-1650 cm^-1, corresponding to the C=O stretching vibration. The exact frequency of absorption depends on the type of carbonyl group and its environment.
Nitrile and Amine Functional Groups
Nitriles and amines are two common types of nitrogen-containing functional groups. Nitriles are characterized by a strong absorption at around 2250-2200 cm^-1, corresponding to the C=N stretching vibration. Amines, on the other hand, show a strong absorption at around 3500-3300 cm^-1, corresponding to the N-H stretching vibration.
The IR spectrum is a powerful tool for identifying functional groups within molecules. By analyzing the IR spectrum of a compound, chemists can determine the presence of specific functional groups, which is essential for understanding the compound's structure and properties.
What is the purpose of IR spectroscopy in organic chemistry?
+
IR spectroscopy is used to identify functional groups within molecules, which is essential for understanding the compound’s structure and properties.
What are the main regions of the IR spectrum?
+
The IR spectrum is typically divided into several regions, including the O-H, N-H, and C-H stretching vibrations (4000-2500 cm^-1), the C=C, C=O, and C=N stretching vibrations (2000-1500 cm^-1), and the C-H, C-O, and C-N bending vibrations (1500-1000 cm^-1).
How do you identify a carbonyl functional group in an IR spectrum?
+
A carbonyl functional group is characterized by a strong absorption at around 1800-1650 cm^-1, corresponding to the C=O stretching vibration.
